Mercury and Aluminium (3)
In this experiment larger amounts of mercury, gallium, and aluminium were used.
 |
 |
 |
 |
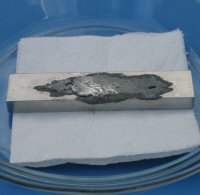
When the mercury was first applied to the aluminium it instantly mixed with the liquid gallium to form a dirty grey liquid, which soaked into the aluminium. I was then suprised when clean shiny mercury came out of the side of the aluminium some time later. This is shown in the first image in the following sequence.
 |
 |
As the reaction had slowed down I applied a few drops of water in the first image in the next sequence and then a lot more water in the second image.
 |
 |
Once the water had seemed to stop doing anything I washed the aluminium oxide off to reveal the aluminium below it. Quite a lot of the aluminium oxide was taken out of the picture during the washing.
The aluminium was then left over night for around 12 hours. When I returned in the morning the aluminium looked quite different. Please note that the powder in the following image was not altered in anyway. I didn't spread the powder out for the sake of the photo.
| Back | Home |

