Mercury and Aluminium (1)
The following sequence of images show the results of applying a tiny amount of gallium to the aluminium (marked with an arrow in the first image) and then applying a small blob of mercury. As you can see the in the third image, the mercury effectively soaks into the aluminium. In the fourth image the aluminium starts to oxidize and flake off.
 |
 |
 |
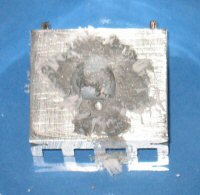 |
The reaction in the images above starts off quite quickly, but slows down after 30 minutes. The soaking of the aluminium usually takes around 5 minutes with the flaking starting after 10 minutes.
Once the reaction looked to have slowed down I decided to remove the visible mercury and observe the results. The following sequence shows what happened. The first image shows the aluminium with the mercury removed, but the aluminium continues to oxidize and grow very thin strands of aluminium oxide. In the second picture I brushed the aluminium oxide off so I could observe it growing again. The third picture shows the aluminium oxide that grew back. The time between the brushing the oxide off and it growing back is probably around 15 minutes.
 |
 |
 |
To speed the process up I decided to put the aluminium heat sink into a jar of water (fully submerged). I didn't phtograph the results, but the reaction was very fast (less than 5 minutes) and it looked as if the aluminium was falling apart. When the reaction came to an end the aluminium was removed from the water and photographed.
The image shows the damage that the mercury, followed by water, did. After watching the whole process I was suprised just how little damage was done. The amount of aluminium oxide released looked to be quite big and so I expected more damage to the aluminium.
| Back | Home |
